The Future of R&D in Pharmaceutical Industry in 2025
EMBA09 team:
Murat Akguc
Ruth Donners
Peter Friedl
Susan Liu
Work under construction. In case you have any questions, additions or comments, please do not edit these pages, but you are more than welcome to contact us.
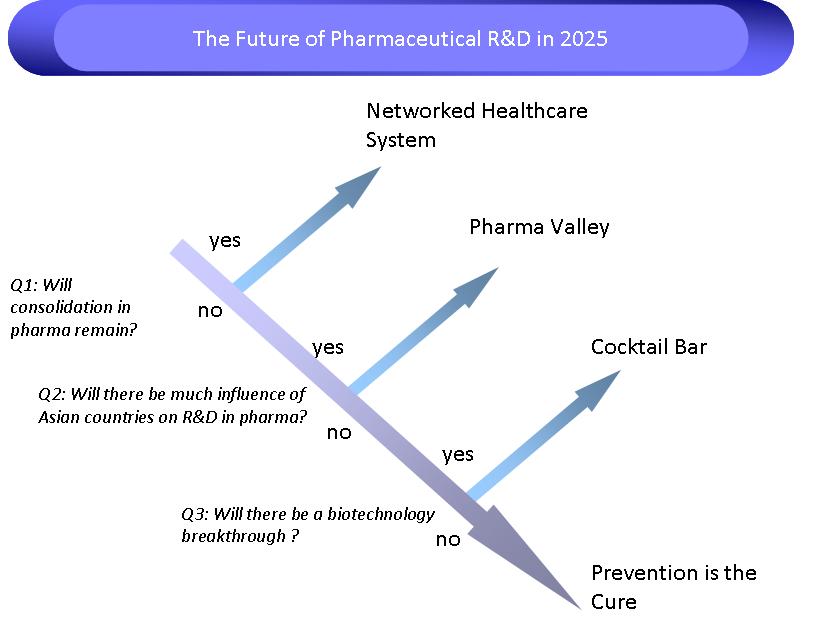
Scenario tree
In the picture below the scenario three leading to the four different scenario's we have identified is shown. In the next section the scenario's are described more elaborately.
Scenarios
Below the 4 developed scenario's can be found, just click on the link to get the full description.
Cocktail bar: personalised medicine
Selfcare scenario, with personalised medicine and focus on prevention, mainly driven by ageing population
Networked healthcare system
Scenario wherein the healthcare system is more integrated, shorter lines between pharma, medicine, insurance companies, which gives pharma incentive to focus more on market pull than technology push
Pharma Valley
Big pharma companies won't be anymore, and a whole scale of specialised smaller R&D companies will emerge
Prevention is the cure
R&D of pharma will go to put part of its focus on establishing health claims for preventative formulations, like food, neutraceuticals and supplements
Research Questions
- Will the system of blockbusters continue? Ie. no more blockbusters in the future, even focus on generics, or are there still enough other diseases to cure with one type of drug or without simply incrementally improving existing drugs?
- Move to emerging markets (subquestions: what is government stance, how is local infrastructure (ie. can you get the drugs to the people?)?
- What are regulatory issues regarding local registration of the drug?
- Will medicine stay a mass product or will personalised medicines become prevalent?
- Will pharmaceutical industry as it is today stay profitable?
- Is there going to be a breakthrough in health?
- Will preventative measures be implemented instead of current focus on care once ill?
- Will pharma R&D stay consolidated or become more fragmented?
- Will there be structural reform in health industry?
- Will pharma industry become less technology push and more market pull?
- Generics vs. Patents? What will the IP landscape be?
Driving forces
Edited existing ones
• Increasing potential to grow based on new available technology
• Struggling old world economy
• Increasing Empowerment of Consumers in Healthcare System
• Continuously growing demand for new medical applications
• The increasing gap between developed, emerging and poor economies
• The increasing globalization of markets
• Power of the United Nations
• Legal Restrictions for Biotech increasing in certain countries, decreasing in others
Added new ones
• IP rights
• Logistics/Distribution
• Illnesses of global importance/Pandemics
• Research budget in pharmaceutical industry
• Effect of development of health care system in emerging market countries to existing pharmaceutical industry
• Unification of regulatory approval
• Increasing Competitiveness through innovation in biotechnology
• Changing business models for pharmaceutical industry
Systems diagram
Moving from chaos to relief
Resources
- [1] Top Trends in Healthcare, Medicine and Pharmaceuticals - What's Next
- [2] 10 Major Healthcare / Pharmaceutical Trends -Jim Carroll's Blog
- [3] Pharma 2020: Virtual R&D "Which path will you take?" - PricewaterhouseCoopers
- [4] OECD International Futures Project on “The Bioeconomy to 2030: Designing a Policy Agenda” - OECD