Difference between revisions of "Scenario BBM"
| Line 18: | Line 18: | ||
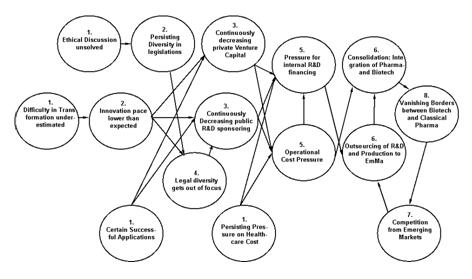
==Scenario Diagram== | ==Scenario Diagram== | ||
[[Image:BBM small.JPG]]<br>Click for larger image: | [[Image:BBM small.JPG]]<br>Click for larger image:[[Biotech Business Matures - Scenario Diagram]] | ||
Revision as of 16:48, 2 December 2005
Biotechnology for Medical Applications in 2015 - Scenario BBM: Biotech Business Matures
The explosion has not taken place. Major players in Biotech as well as private and public investors in medical biotech research had expected a revolution in medical applications, grace to a vastly increased knowledge and fundamental research base and thus a vast universe of opportunities for new applications with shrinking R&D cost. This has not happened. Developing successful applications upon the vast knowledge base in biotech turned out to be much more difficult and much more expensive than expected. The rate of innovation finally didn’t turn out to be higher than it was in the years and decades before with classical pharmaceutical R&D. In addition, development periods for new drugs generally even became longer, due to increased security concerns and thus stricter approval practices by both, FDA and EMEA. Starting from around 2009, biotech was no longer considered a hot topic by the venture capital community, particularly in the US. In the Industry for medical application the border between classical pharma and biotechnology began to vanish. Nowadays (2015), this border has virtually disappeared.
Cost pressure in healthcare inclusive the cost pressure for drugs and medical applications persisted. Efforts of the industry, to bring drug prices out of the public focus as being one of the major cost drivers in healthcare, failed. Healthcare costs now make around 30% of the US government spending, and in other countries there is not much difference. Governmental efforts in several countries to transfer a bigger part of the healthcare cost from the public household to private individuals met considerable resistance and could only be conducted to a small degree. These measures only had a slight impact on the development of healthcare costs. In addition, structural problems as well as adverse incentives in healthcare remained partly unsolved. As a consequence, pressure on the drug industry to reduce drug prices increased. Especially in the US, the government was no more willing, to accept that drug prices in the country exceeded those in other parts of the world by significant percentages. Parallel import had therefore been made possible. The same was the case in those European countries where drug prices exceeded those in the rest of the world. Health insurers and politicians at the same time enhanced the incentives for consumers to ask their doctors to prescribe generics wherever possible which was followed to a large degree. All this had the consequence that margins on drugs in general got smaller and cost pressure for the pharma- and biotech industries increased. Drug production and R&D activities began to get transferred to emerging markets. But still, profitability of products remained tight.
Governments decreasingly saw biotechnology as the panacea to solving all the economic problems of their country. Biotechnology in medical applications was more and more becoming an industry like any other. Profitability established itself about on the level that previously had already been reached by most pharma multinationals but remained under steady pressure.
Private Equity and Venture Capital investors decreasingly considered biotechnology as the central hot investment topic. Other topics like nanotechnology began to replace biotechnology as the most hyped investment themes from 2006 onwards. Today, in 2015, the investment community already fears the burst of a nanotech bubble. Increasingly, companies had to finance R&D activities in biotech out of their own pockets – which increased the pressure on the industry to consolidate.
Ethical problems remained unsolved for a long time and the debate still keeps popping up from time to time. The 2005 presidential veto in the US against stem-cell research funding with public funds persisted and inhibited the implementation of a corresponding legal framework. For some time, both, the US biotech industry as well certain leading US economists feared that they could fall back against countries like the UK or south Korea – which had a much more permissive legal framework. However this discussion calmed down pretty soon. Transformation of basic research - including basic research in stem cell cloning - into marketable medical applications had proved to be much more difficult than expected and more difficult to plan than expected. In fact, some successful applications have been developed. A successful cure for bird flu was launched in 2009 and a new MS drug (which is still not able to heal the disease but which is able to delay the symptoms for much longer) was launched shortly after. But the high hopes set into the industry were not fulfilled. Common worldwide notion today is, that the innovation pace is not higher than the one we have been observing in the pharmaceutical industry for the last 20 years. Development costs still are around € 1billion for a successful application. Additionally only one project out of eight is successful. For these reasons, the ban of publicly funded stem-cell research had gotten out of focus as a major barrier hindering economic growth in the US. In other countries, there was no major boom in successful medical applications based upon stem cell research – the revolution is still to happen.
Competition among countries in biotechnology can be considered as healthy. None of the G9 (former G7) and G13 (former G10) countries today considers biotechnology as their only key topic for economic development. The same is the case with most of the important emerging markets. In view of the fact that governmental sponsoring was producing encouraging results in basic research but that successful transformation of these into successful application and hence into jobs and economic growth did not take place at the pace that governments had hoped, they stopped pumping funds aggressively into biotech research. India and China became centres for outsourced applied R&D, they built up considerable manufacturing capacity for biotech drugs and built up an increasingly competitive local pharmaceutical industry. However, this is considered nowadays as a normal consequence of both globalisation and the trend towards outsourcing to these areas of the world. To summarize, support of biotechnology is no longer the first priority of governments in most of the important economic areas of the world.
Borders between biotech and classical pharma have vanished. Biotechnology majors have expanded into the business with pharma generics. Likewise former pharma multinationals have built up vast biotech operations. Today it is no longer an issue whether a drug is based on biotechnology or whether it is an outcome of classical pharma research. The Biotech / Pharma industry grows with about 8-10% per year which is similar to the Growth Rate of Pharma between 2000 and 2005.
Scenario Diagram
Click for larger image:Biotech Business Matures - Scenario Diagram